The pharmaceutical and biotechnology industries are increasingly reliant on robust data collection and analysis to ensure the safety and efficacy of clinical trials. A critical component of this process is the creation and maintenance of comprehensive monitoring reports. These reports provide a detailed account of the trial’s progress, highlighting key metrics, identifying potential issues, and informing decision-making. A well-structured monitoring report template is essential for maintaining transparency, accountability, and ultimately, the success of clinical trials. This article will delve into the key elements of a robust monitoring report template, exploring its purpose, structure, and best practices. Monitoring Report Template Clinical Trials is more than just a document; it’s a vital tool for regulatory compliance, stakeholder communication, and optimizing trial outcomes. It’s a strategic asset that can significantly improve the efficiency and effectiveness of clinical research.
The clinical trial process is inherently complex, involving numerous variables and potential risks. Monitoring reports serve as a central point of reference, consolidating data from various sources – patient assessments, laboratory results, adverse event reports, and clinical observations – into a single, easily digestible format. Without a standardized reporting system, it becomes challenging to track progress, identify deviations from the protocol, and proactively address potential problems. Early detection of issues allows for timely intervention, minimizing risks to participants and accelerating the timeline for results. Furthermore, transparent and accurate reporting builds trust with regulatory agencies, sponsors, and other stakeholders. The ability to demonstrate adherence to protocol and a commitment to patient safety is paramount in today’s regulated environment. The cost of delays or failures due to inadequate monitoring can be substantial, impacting both financial resources and the reputation of the research institution.

A comprehensive monitoring report template typically includes several key sections. Each section is designed to address a specific aspect of the trial’s progress. Let’s examine some of the most important components:
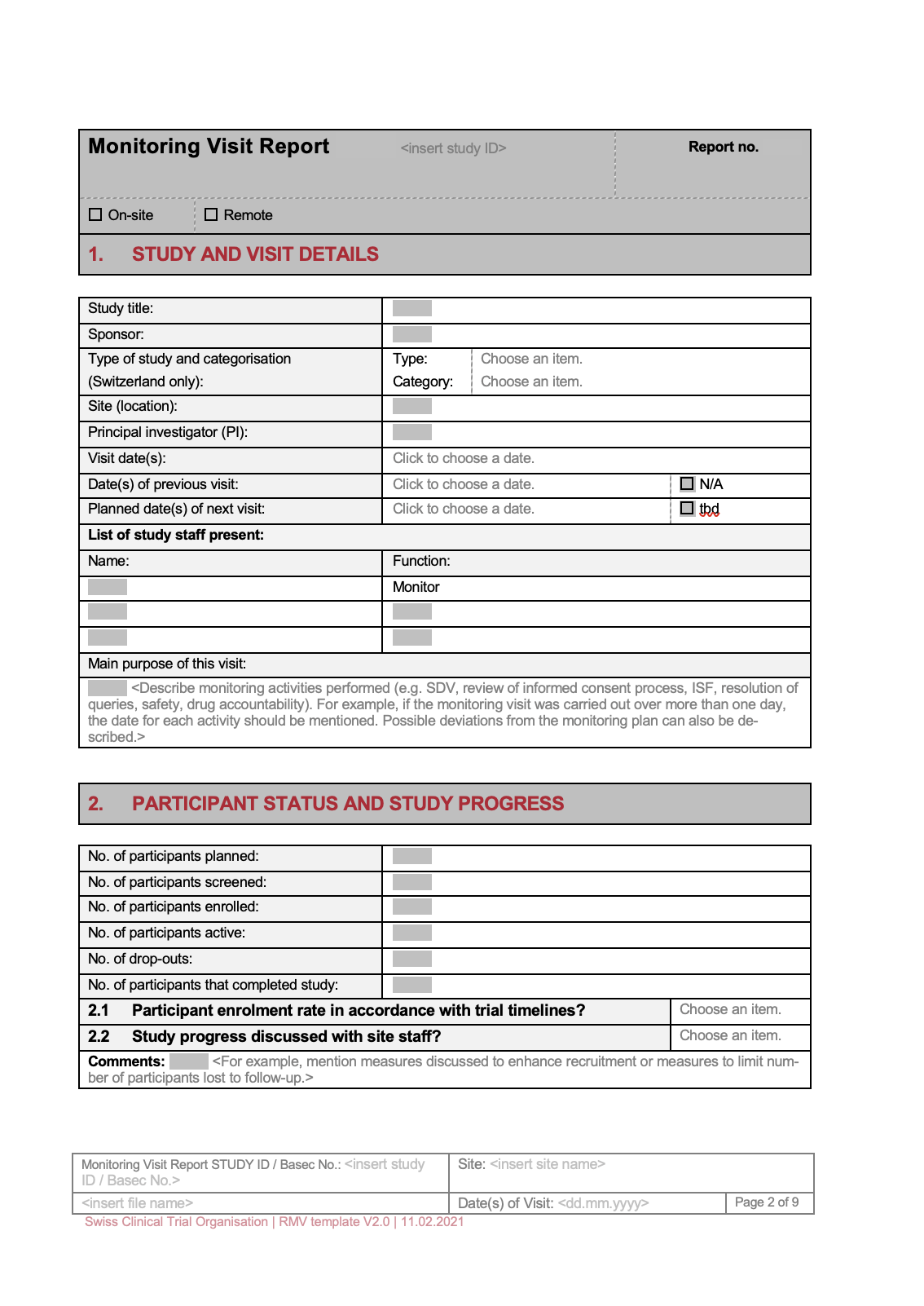
This initial section provides a concise summary of the trial’s purpose, design, and key objectives. It should clearly state the intended population, the primary endpoints, and the overall hypothesis being tested. It’s crucial to reiterate the Monitoring Report Template Clinical Trials objectives at the beginning of this section. This section also includes a brief description of the study protocol and any relevant background information. Key data points to include are the study start and end dates, the number of participants enrolled, and the primary outcome measures.
This section meticulously details the data collected from each participant. It covers a wide range of information, including demographic details, baseline characteristics, medical history, and concomitant treatments. Detailed records of adverse events (AEs) are essential, along with their severity and management. Data on vital signs, laboratory values, and physical examinations are also typically included. The template should facilitate easy data entry and analysis, utilizing standardized forms and data validation rules. Proper documentation of patient consent and informed consent is a critical element here.

This section focuses on the observations made by clinical investigators and monitors during the trial. It includes detailed notes on patient behavior, adherence to the protocol, and any unusual events. This section is particularly important for identifying potential safety concerns or deviations from the protocol. It’s vital to maintain a consistent and objective record of observations, avoiding subjective interpretations. The template should allow for the recording of observations related to patient symptoms, treatment response, and any changes in condition.
Detailed laboratory data is a cornerstone of the monitoring report. This section includes results from routine blood tests, urine tests, and other relevant laboratory assays. It’s important to clearly identify the test, the date of the result, and the corresponding values. Any significant deviations from the expected range should be documented and investigated. The template should facilitate the generation of standardized reports for laboratory data, ensuring consistency and comparability across different sites.
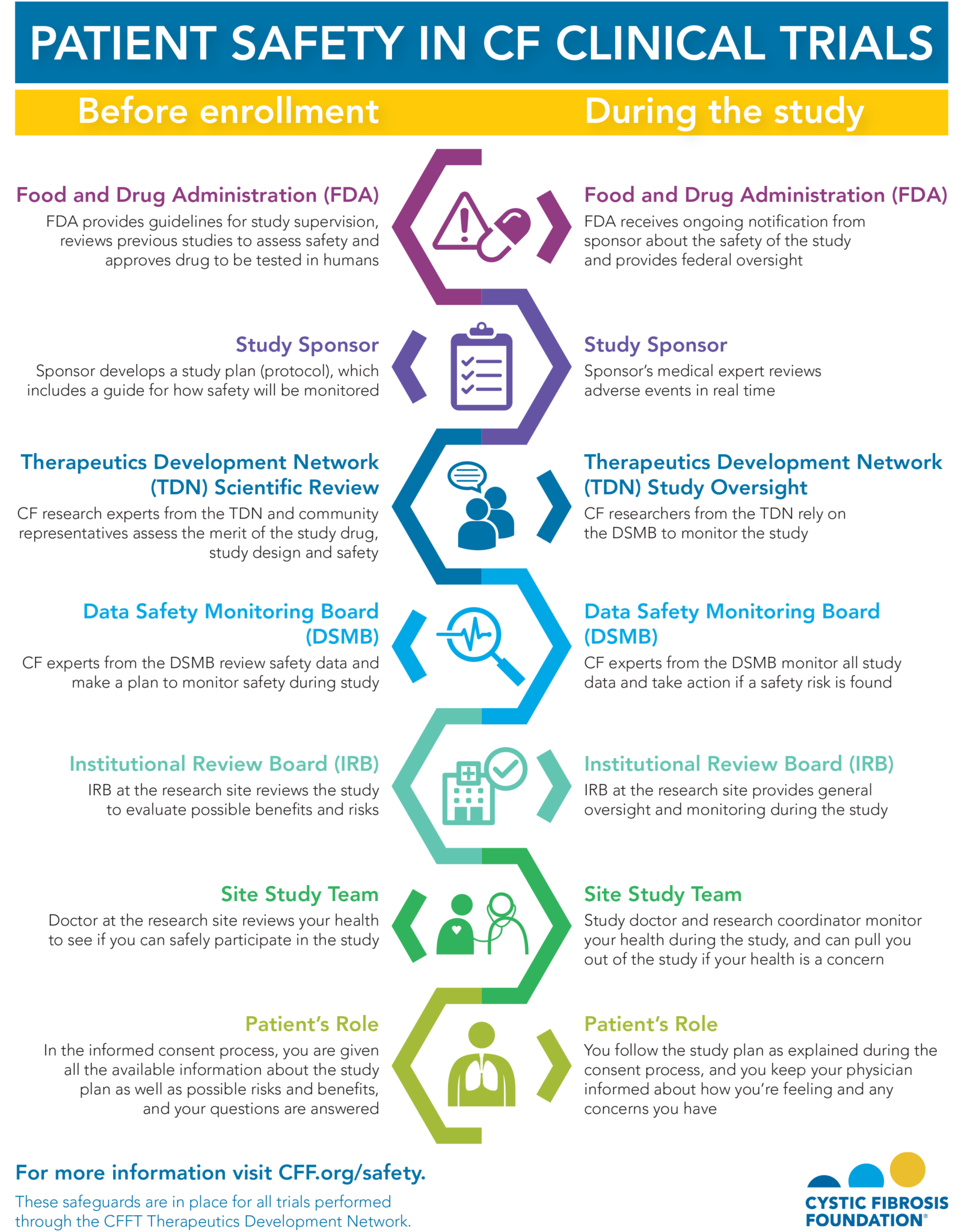
A comprehensive AE reporting section is vital for identifying and managing potential safety risks. This section requires detailed information about all adverse events, including the date of onset, severity, and management. It should include a standardized form for recording AE information, ensuring consistency and facilitating data analysis. The template should allow for the tracking of AE types, severity, and potential causality. Regular review of AE reports is essential for identifying trends and implementing preventative measures.
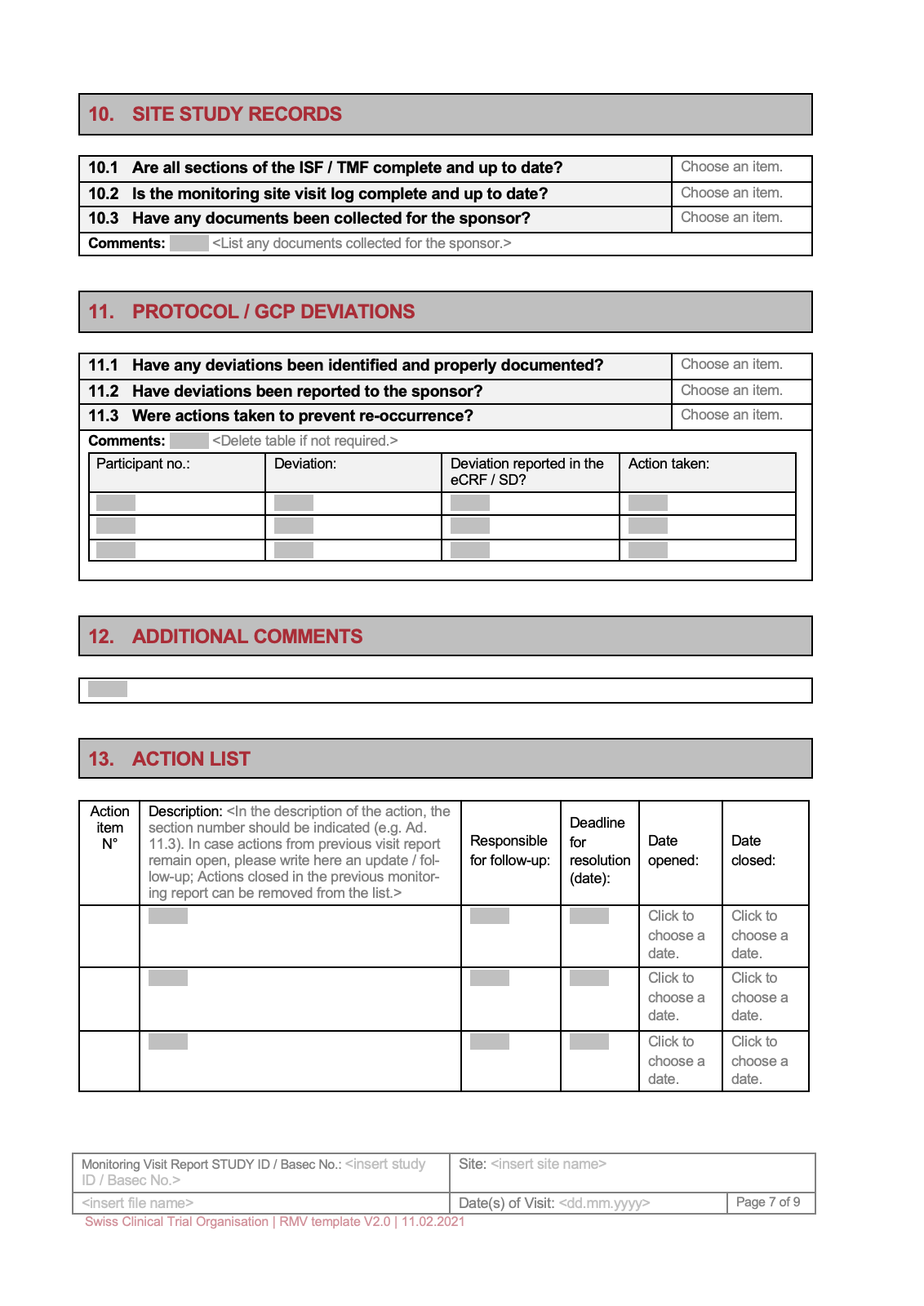
This section documents any deviations from the protocol, including changes in study design, treatment protocols, or data collection procedures. It’s important to clearly explain the rationale for the deviation and its potential impact on the trial. The template should facilitate the tracking of protocol changes and their effect on the data. Proper documentation of protocol deviations is crucial for maintaining regulatory compliance and ensuring the integrity of the data.

This section provides a summary of the statistical analysis performed on the data and highlights any data quality issues. It should include information on the statistical methods used, the sample size, and the confidence intervals. It also includes a discussion of any potential biases or limitations of the data. The template should facilitate the generation of statistical reports and the identification of potential data quality issues.
Modern monitoring report templates are increasingly leveraging technology to enhance efficiency and accuracy. Electronic data capture (EDC) systems, data management software, and automated reporting tools can streamline data collection, reduce errors, and improve data quality. Cloud-based platforms allow for seamless data sharing and collaboration among study sites. Furthermore, data visualization tools can help researchers identify trends and patterns in the data more quickly. The use of artificial intelligence (AI) and machine learning (ML) is beginning to be explored for automated data analysis and anomaly detection. These technological advancements are transforming the way monitoring reports are created and maintained.
Several best practices can significantly improve the effectiveness of monitoring reports. These include:
Monitoring reports are an indispensable tool for ensuring the success of clinical trials. A well-structured and meticulously documented monitoring report template is essential for maintaining transparency, accountability, and regulatory compliance. By carefully considering the key components outlined in this article, researchers and sponsors can create reports that effectively communicate trial progress, identify potential issues, and ultimately, contribute to the advancement of medical knowledge. The continued evolution of technology is further enhancing the capabilities of these reports, making them more efficient and insightful. Ultimately, a robust monitoring system is a critical investment in patient safety and the integrity of clinical research.
The clinical trial landscape is constantly evolving, demanding sophisticated data management and reporting capabilities. The monitoring report template, when implemented effectively, provides a critical framework for navigating this complexity. By prioritizing data quality, adhering to regulatory guidelines, and leveraging technology, researchers can ensure that clinical trials deliver meaningful results that benefit patients. The ongoing integration of data analytics and AI promises to further refine monitoring practices, leading to improved trial design, faster results, and ultimately, a more efficient and effective healthcare system. Continuous improvement and adaptation of the monitoring report template are essential to remain competitive and responsive to the changing needs of the industry.